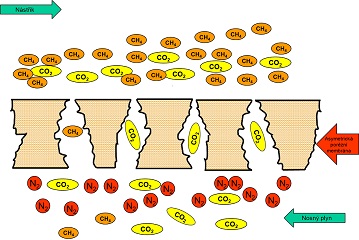
Recently, the number has been growing of industrial applications for the separation of gases and vapours, working on the basis of membrane processes. Gas separation is performed with porous or nonporous membranes. With porous membranes, the separation principle is based on so-called sieve effect, using various sizes of the membrane pores and various sizes of gas molecules to be separated (Fig 1).
In case of nonporous membranes, the separation principle is based on so-called solution–diffusion mechanism, for which
P=D x S
,
where P is the permeability coefficient, D is the diffusion coefficient and S is the solution coefficient.
Based on this mechanism, the separation of individual components in the mixture is only effective in case that there is an ordinal difference between these components in at least one of the coefficients (diffusion or solution) (Fig 2).
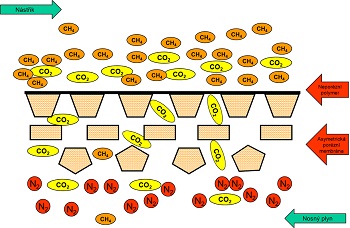
Total transport of the mass through the membrane is caused by the driving force (or its gradient). In gas separation, this driving force is a pressure difference on both side of the membrane (<8MPa). The pressure difference can be created either with vacuum on the other (permeate) side of the membrane or with a carrier gas, using overpressure on the upstream side of the membrane.
In the solution–diffusion mechanism, it is supposed that there is sorption of molecules on the upstream side of the membrane first, then molecules diffuse through the membrane and finally they desorp on the permeate side of the membrane into the carrier gas or vacuum. The controlling step of separation is either diffusion or sorption of the component which is the slowest in a particular membranes or it adsorbs least.
The permeability of a material for gases and vapours is a characteristic technological parameter which is determined experimentally on the basis of various direct or indirect techniques and it is usually given in the units of Barrer, where
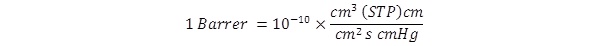
The efficiency of separation of mixtures of gases and vapours is given e.g. using so-called separation factor, which is defined as the quotient of the permeability coefficients of individual pure components of the mixture:
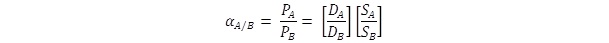
The ratio of the diffusion coefficients DA/DB defines the differences of the molecule sizes of permeating gases; the ratio of the permeability coefficients SA/SB reflects the rate of interactions between the molecules of penetrants and membrane or the solubility of individual components in the membrane.
Another possible method to express the efficiency of separation is the separation factor Sc(AB), defines as:
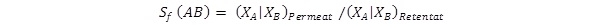
where X is the molar fraction of a component in the permeate or retentate.


































 EUROPEAN UNION
EUROPEAN UNION